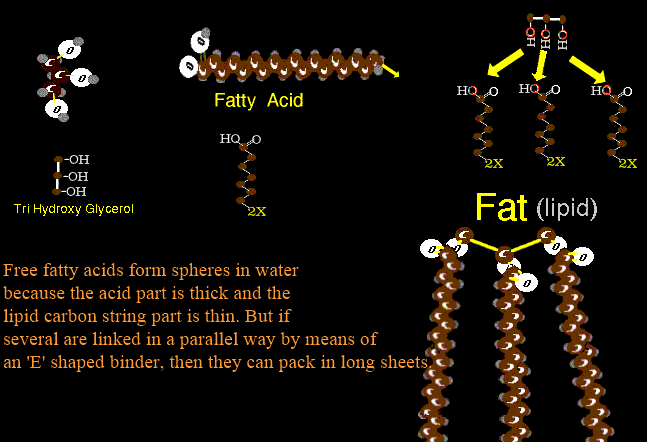|
|||
|
Lipids It ain't over til ...
Fats, oils, things greasy - those are lipids. One key property is repulsion of water. Long chains of carbon -C-C-C-C-C-C-C=C-C-C- with all the free carbon handles taken up by hydrogen are the common theme - carbon 'saturated' with hydrogen (not shown). Carbon, with four handles, can bind four hydrogens (each hydrogen has one handle). Occasionally carbon will use two handles to link up such as -C-C=C-C- which it can do with other carbons or with oxygen as O=C=O (CO2). Such carbon is unsaturated (could take more hydrogen). The single carbon C-C-C-C bonds form a straight line. They pack well and with little space attract strongly side to side as solids. The unsaturated links as -C=C- are bent and look like this _._._./ causing the chain of carbons to zig and zag thus not packing tightly and with far less side to side contact forming more fluid groups as oils. There are such pure long chains with minimal else such as a rare end CCCCCCC-OH that joins with a rare CCCCCCC-C=O -OH to yield ...CCCCCCCCCCOO-CCCCCCCCCCCC... which pack very tightly (wax). But cells are a water environment. There has to be compromise and a way to deal, in a water
way, with the oils - so they have acid ends" This carbon chain with the acid cap is a "fatty acid". OR... if the lipid parts all stick together in a central core with the acid parts to the outside interacting with water, forming "micells" (meaning mini- icky-cells, though I could be wrong). Well, micells are cute, but kind of limited. fatty acids can be transported that way - as spherical blobs, but to what end?
The three carbon unit having three alcohol -OH hook spots will join three fatty acids together like three keys on one key link with the key carbon chains fairly parallel. This is called 'FAT' which is short for 'Fat chance' - though I could be wrong about that. Notice also that although the fatty acid carbon chains may be many lengths, the lengths are always of even numbers of carbons as the chains are built up via units of Acetyl-CoA. Fat is densely packed highly saturated (with hydrogen) carbon. Think of all that hydrogen that could be put on the credit card (NAD) for burning or the carbon that can be burned by O2 for direct cell money - energy. Fat is very dense and efficient energy storage and carbon storage. It also makes good insulation, so put most of it on the outside where it will do the most good. It's just storage, so sculpt it to attract attention. Oh, speaking of storage, why not store other oil soluble things there? OK. Oil soluble vitamins go there for use when low, as do many hormones which - designed to work in oily cell membrane environments - are pretty slippery themselves. Thus women with no body fat have hormone adjustment problems as they lack fat storage space. Anesthesia gasses are nearly all fat soluble (they DO work on cell membranes in the oily environment there). Therefore, folks with lots of fatty storage space will take a long time to wake up as the anesthesia saturates the fat and leeches back out rather slowly. It ain't over until the fat layer synchs.
|
| [P.O. Home] [Topics] [Muscle] [Basic Science] [Lipids] |
|
|
|
|